-
Looking for HSC notes and resources? Check out our Notes & Resources page
Lewis structure (1 Viewer)
- Thread starter highshill
- Start date
oh if ur just asking how to draw lewis structures use this:
To draw them follow the steps:
Calculate the total amount of valence electrons
Most electropositive element is used as the centre atom (except hydrogen)
Place other atoms around it using single bonds
Populate the outer atoms with 3 lone pairs of electrons each if not a hydrogen
Assign remaining electrons to central atom
Modify shape to satisfy octet rule
Minimise formal charges where possible (look up on google how to do it)
To draw them follow the steps:
Calculate the total amount of valence electrons
Most electropositive element is used as the centre atom (except hydrogen)
Place other atoms around it using single bonds
Populate the outer atoms with 3 lone pairs of electrons each if not a hydrogen
Assign remaining electrons to central atom
Modify shape to satisfy octet rule
Minimise formal charges where possible (look up on google how to do it)
Fizzy_Cyst
Well-Known Member
Valence Shell Electron Pair Repulsion (VSEPR) helps describe the shape.How can you tell the molecular shape of a atom.
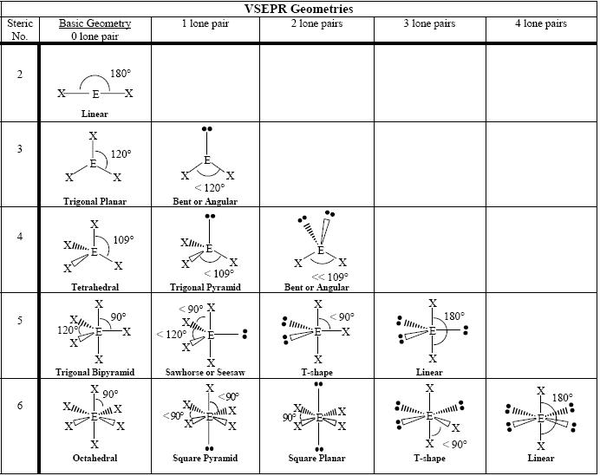
It's basically commiting the main "electron pair" shape to memory and then determining which shape the molecule is from looking at number of bonding and non-bonding pairs
