splendid haha, went from people telling me I was wrong because of something to do with ACETIC acid to now hopefully being right - bumps my short answers up by 2 marks which is always niceConfirm, I had something like that
-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
General thoughts: HSC chemistry 2015 (1 Viewer)
- Thread starter hawkrider1
- Start date
Librah
Not_the_pad
Why was that worth 4 marks?
3(0.1)(0.025)/0.0415=0.181 M
3(0.1)(0.025)/0.0415=0.181 M
The only reason I'm thinking this may be wrong is by looking at it logically - if 25mL of 0.1mol citric (weak) acid needed 41.5mL of NaOH (strong), it would make sense that the NaOH would be more dilute than this acid? Correct me if Im wrong hereI got 0.181 (3sf.) lol
Was the question something like 25mL citric at 0.100mol/L reacts with sodium hydroxide and avg sodium hydroxide was 41.5mL?
kawaiipotato
Well-Known Member
- Joined
- Apr 28, 2015
- Messages
- 462
- Gender
- Undisclosed
- HSC
- 2015
That sounds logical. But they gave amount of citric needed to neutralise it anyway so wouldn't this already mean it takes into account the weakness of it?The only reason I'm thinking this may be wrong is by looking at it logically - if 25mL of 0.1mol citric (weak) acid needed 41.5mL of NaOH (strong), it would make sense that the NaOH would be more dilute than this acid? Correct me if Im wrong here
C6H8O7 + 3NaOH ---> Na3C6H5O7 + 3H2O
nC6H807 = 0.1*0.025 = 0.0025
nNaOH = 0.0025*3 = 0.0075
[NaOH] = 0.0075/0.0415 = 0.108mol/L
I have a feeling I just lost out on 4 marks now
Librah
Not_the_pad
watThe only reason I'm thinking this may be wrong is by looking at it logically - if 25mL of 0.1mol citric (weak) acid needed 41.5mL of NaOH (strong), it would make sense that the NaOH would be more dilute than this acid? Correct me if Im wrong here
Dw guys you are right I think I've just got this all mixed up
Librah
Not_the_pad
Citric acid has 3 Carboxylic acid groups iirc? Donates 3 protons.
Ekman
Well-Known Member
- Joined
- Oct 23, 2014
- Messages
- 1,611
- Gender
- Male
- HSC
- 2015
Sorry, I just looked through the question again and I got the same answer as librah (I remember the multiplying the moles by 3 to get 0.0075, and dividing by 0.0415 to get 0.181).splendid haha, went from people telling me I was wrong because of something to do with ACETIC acid to now hopefully being right - bumps my short answers up by 2 marks which is always nice
I must of remembered 0.006 from some other question I did last night.
Edit: I remembered it from the 2014 hsc paper I did last night, where the answer to question 30 a was 0.005
Last edited:
for that citric acid one i forgot it was triprotic thought it was mono for some reason but did the working out and got the answer off by a 3 factor, what do you think ill get for it, its bullshit that was a 4 marker, 2 or 3 marker at most man
CookieMan999
New Member
- Joined
- Jan 30, 2014
- Messages
- 7
- Gender
- Male
- HSC
- 2015
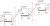
I picked B for the polymer in Q11 because as in glucose, two hydroxyl groups (OH) condense to expel a H2O and leave only ONE oxygen atom to form the link. None of the other options display this.
D looked correct at first but having an oxygen on both sides would mean that they had condensed to form H2 (similarly for C, 2 oxygens are in the middle for the link instead of one from condesation). A proper answer should have had D but with one less O on one side.
Last edited:
Im probabaly thinking way too much into it, but maybe it wasnt necessarily H2O that was condensed out, as condensation polymerization can refer to the release of any small molecule, not just H2O, maybe it could have been H2 or methanol instead although, but otherwise, all the options on there are technically wrong, could just send some complaints in about it tbhView attachment 32594
I picked B for the polymer in Q11 because as in glucose, two hydroxyl groups (OH) condense to expel a H2O and leave only ONE oxygen atom to form the link. None of the other options display this.
D looked correct at first but having an oxygen on both sides would mean that they had condensed to form H2 (similarly for C, 2 oxygens are in the middle for the link instead of one from condesation). A proper answer should have had D but with one less O on one side.
BillKyriakos
New Member
- Joined
- Jun 18, 2014
- Messages
- 13
- Gender
- Male
- HSC
- 2015
What do you guys think an 85 would align to?
Yeah I think you're thinking way into it lol. BOS generally never extends into condensation with H2 or methanol product.Im probabaly thinking way too much into it, but maybe it wasnt necessarily H2O that was condensed out, as condensation polymerization can refer to the release of any small molecule, not just H2O, maybe it could have been H2 or methanol instead although, but otherwise, all the options on there are technically wrong, could just send some complaints in about it tbh
This won't be helpful but tbh nobody can say definitively. Even though the general consensus seems to be that it was an "easy" paper, we still need to see how the state goes holistically. Also mark alignment can change dramatically from year to year, from memory, 85 went to 95 in one year whilst 90 went to 94 in another year. So yeah, it's a little bit hard to predict.What do you guys think an 85 would align to?
BillKyriakos
New Member
- Joined
- Jun 18, 2014
- Messages
- 13
- Gender
- Male
- HSC
- 2015
That's fair. Thanks for the responseThis won't be helpful but tbh nobody can say definitively. Even though the general consensus seems to be that it was an "easy" paper, we still need to see how the state goes holistically. Also mark alignment can change dramatically from year to year, from memory, 85 went to 95 in one year whilst 90 went to 94 in another year. So yeah, it's a little bit hard to predict.
iforgotmyname
Metallic Oxide
- Joined
- Jun 16, 2015
- Messages
- 731
- Gender
- Male
- HSC
- 2015
For the industrial question, Moles wereI got 10.6?
0.5 + 1 -> 2.5. Where the one with 0.5 mole has 2 in front and rest has 1 in front
It said the one with 0.5 moles had change (increase) in concentration of 0.36 moles.
This will mean that the one with 1 mole will increase by 0.36x2 (due to the ratio being 2:1 between 0.5 molar and 1molar substance on lhs) This will also mean that (00.36x2) 2.5 moles product will be consumed to make the lhs reaction.
You divide everything by 2 thus yielding you
0.43 + 0.86 -> 0.89 thus equilibrum constant will be around 5.59
Idk is this right or did I fk up the molar ratio
becauseiambored
New Member
- Joined
- Oct 23, 2015
- Messages
- 3
- Gender
- Male
- HSC
- 2015
Clean full pdf version of 2015 Chemistry HSC Paper.
https://www.dropbox.com/s/srhwrj25tmu7jbj/hsc_chem_2015.pdf?dl=0
https://www.dropbox.com/s/srhwrj25tmu7jbj/hsc_chem_2015.pdf?dl=0
BlueGas
Well-Known Member
- Joined
- Sep 20, 2014
- Messages
- 2,439
- Gender
- Male
- HSC
- N/A
What question was this again? I remember I got the same answer and I left the answer as 0.18If i left my answer as 0.1807 (stressing out bout this) will i lose a mark as i think its one too many sf
NaOH concWhat question was this again? I remember I got the same answer and I left the answer as 0.18
