genril
Banned
- Joined
- Aug 6, 2007
- Messages
- 86
- Gender
- Male
- HSC
- 2008
Posting it here rather than the bio or chem forum because it is kind of general.
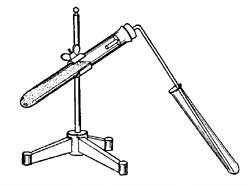
"Carbon Dioxide (CO2) can be made by adding Hydrochloric Acid (HC1) to Marble Chips (CaCO3). This is the method you will use to generate the gas. How you collect the gas, dissolve it and test for changes in pH is to be determined by the group or individual"
Now, How do I collect the gas? I have never done anything like this before, and if it was upto me I would be getting a straw and blowing the carbon dioxide into the beakers (I will be asking if I can do this, it works ).
).
Thanks.
Oh and by the way, this is for the bio experiment where we have to demonstrate the effect of dissolved carbon dioxide on the pH of water.
"Carbon Dioxide (CO2) can be made by adding Hydrochloric Acid (HC1) to Marble Chips (CaCO3). This is the method you will use to generate the gas. How you collect the gas, dissolve it and test for changes in pH is to be determined by the group or individual"
Now, How do I collect the gas? I have never done anything like this before, and if it was upto me I would be getting a straw and blowing the carbon dioxide into the beakers (I will be asking if I can do this, it works
Thanks.
Oh and by the way, this is for the bio experiment where we have to demonstrate the effect of dissolved carbon dioxide on the pH of water.
Last edited: