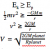So hi guys this is going to be a new thread on which I post helpful tips for the subjects I did during the HSC (listed in the title and my signature), as well as some general advice for the HSC.
I'll be trying to post a few times a week, so check back as I post to see the ways in which I achieved my high ATAR, as well as other achievements such as scholarships (got offered three, so feel free to also post below if you would like to know what types of things that they look for in an application).
If you have any questions about what I write just let me know.
So here's the first tip:
In chemistry, Make sure to include equations in all answers where possible (even if the question does not explicitly ask) as this shows your extended knowledge of the content and is crucial if you are aiming for a band 6 mark. Also, diagrams for molecules that you are describing should be drawn (allows you to show the marker how the intermolecular forces interact within that molecule for example and also this is really important in the polymer section - draw the structure of LDPE and HDPE, it will show the difference in chain branding as shown in the picture below):

(HDPE)

(LDPE)
The reason why there is a disparity in physical properties even though they are made from ethene monomers, between these two structures is because of the chain branching. Just think of it as sticks from a tree, If I have a bunch of straight sticks obviously they are going to stack much more easily and into a small space compared to a bunch of sticks with lots of branches. this means there will more space between the electrons and this will reduce the amount of dispersion forces that can form in LDPE, and the same principle increases it in HDPE as you have to remember these are electric fields causing these forces which must obey the inverse square law. this property is called the stacking ability
this all leads to the difference in melting point, strength, flexibility etc
I'll be trying to post a few times a week, so check back as I post to see the ways in which I achieved my high ATAR, as well as other achievements such as scholarships (got offered three, so feel free to also post below if you would like to know what types of things that they look for in an application).
If you have any questions about what I write just let me know.
So here's the first tip:
In chemistry, Make sure to include equations in all answers where possible (even if the question does not explicitly ask) as this shows your extended knowledge of the content and is crucial if you are aiming for a band 6 mark. Also, diagrams for molecules that you are describing should be drawn (allows you to show the marker how the intermolecular forces interact within that molecule for example and also this is really important in the polymer section - draw the structure of LDPE and HDPE, it will show the difference in chain branding as shown in the picture below):

(HDPE)

(LDPE)
The reason why there is a disparity in physical properties even though they are made from ethene monomers, between these two structures is because of the chain branching. Just think of it as sticks from a tree, If I have a bunch of straight sticks obviously they are going to stack much more easily and into a small space compared to a bunch of sticks with lots of branches. this means there will more space between the electrons and this will reduce the amount of dispersion forces that can form in LDPE, and the same principle increases it in HDPE as you have to remember these are electric fields causing these forces which must obey the inverse square law. this property is called the stacking ability
this all leads to the difference in melting point, strength, flexibility etc
Last edited: