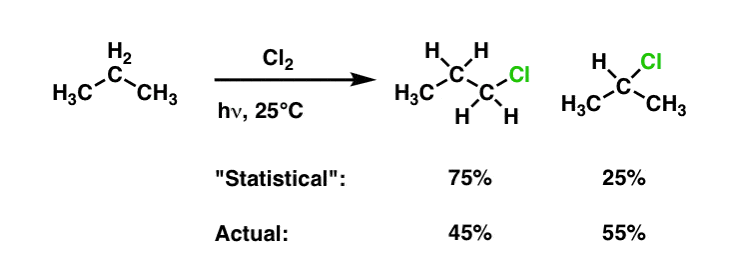
Quick question regarding some reactions, how can you form a primary alcohol from an alkene? eg form 1-propanol from propene. My understanding is that if you were to hydrate propene in the presence of dilute H2SO4 the hydroxyl group will add to the second carbon (by Markovnikov's rule, at least this product will dominate) thus forming 2-propanol which cannot then be transformed into a carboxylic acid for making an ester (as an example). The only other method I can think of is first reacting the alkene with H2 to produce the corresponding alkane and then react that with X2 in the presence of UV light to produce a haloalkane which then can be hydrated to form a specific alcohol. But I am not sure whether to use this process because I dont know if the product of the halogenation of propane (say with bromine) will be 1-bromopropane or 2-bromopropane, in the case of the latter I am then unable to make my primary alcohol that I want for the reaction. Any suggestions would be appreciated!
Last edited: