This is a poorly posed question and you're absolutely right to be confused.
Using Ksp
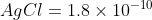
whereas Ksp

So just by using the Ksp, we could infere that Cl- will be precipitated out first. Since the flow chart doesn't demonstrate the production of 2 precipitates due to the addition of AgNO3, we can safely assume
precipitate 1 is AgCl. We also have to assume that exactly the right amount of AgNO3 was added, such that no AgOH precipitated.
Then, we add Ca2+. We know all sulfates are soluble except for Ca2+, Ba2+ and Pb2+. Thus this must be CaSO4. We confirm this using the fact that all hydroxides are insoluble, except for Rule 2, lead and Ca (slightly).
So precipitate 2 is CaSO4, filtrate 2 is a hydroxide solution.
So the answer is C
For reference, the solubility rules:
1) All nitrates and acetates are soluble
2) All Group 1 and ammonium salts are soluble
3) All chlorides, bromides, iodides are soluble except for silver and lead
4) All sulfates are soluble except barium, lead, calcium
5) All hydroxides are insoluble except for Rule 2, barium and calcium (slightly)
6) All carbonates are insoluble except Rule 2

