Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Enthalpy of neutralisation for ammonia (1 Viewer)
- Thread starter synthesisFR
- Start date
synthesisFR
afterhscivemostlybeentrollingdonttakeitsrsly
pLs HeLP
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 441
- Gender
- Undisclosed
- HSC
- 1998
I am not going to do the calculation for you, but I will outline the method. Water formation has nothing to do with this problem. In this neutralisation reaction, we are donating a proton to ammonia. There is no water formation.
To calculate specific heat capacity, we need three pieces of data:
Q, the amount of heat liberated in the reaction, which we don't know.
m, the total mass of the system, which is 110.5 g
ΔT, the change of temperature of the system, which is 53.7 C
Specific Heat capacity C = Q/(m.ΔT)
I'm pretty sure that the ammonia will be the limiting reagent, but you should check this.
You have to work out how many moles of reaction will occur.
Multiply the moles of reaction by the ΔHreaction to get Q
Do you think you can complete the question now?
To calculate specific heat capacity, we need three pieces of data:
Q, the amount of heat liberated in the reaction, which we don't know.
m, the total mass of the system, which is 110.5 g
ΔT, the change of temperature of the system, which is 53.7 C
Specific Heat capacity C = Q/(m.ΔT)
I'm pretty sure that the ammonia will be the limiting reagent, but you should check this.
You have to work out how many moles of reaction will occur.
Multiply the moles of reaction by the ΔHreaction to get Q
Do you think you can complete the question now?
synthesisFR
afterhscivemostlybeentrollingdonttakeitsrsly
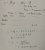

yeah i get all thisI am not going to do the calculation for you, but I will outline the method. Water formation has nothing to do with this problem. In this neutralisation reaction, we are donating a proton to ammonia. There is no water formation.
To calculate specific heat capacity, we need three pieces of data:
Q, the amount of heat liberated in the reaction, which we don't know.
m, the total mass of the system, which is 110.5 g
ΔT, the change of temperature of the system, which is 53.7 C
Specific Heat capacity C = Q/(m.ΔT)
I'm pretty sure that the ammonia will be the limiting reagent, but you should check this.
You have to work out how many moles of reaction will occur.
Multiply the moles of reaction by the ΔHreaction to get Q
Do you think you can complete the question now?
But the point is isnt the moles in the equation always meant to be the number of moles of water produced for a neutralisation reaction? The second picture is from the theory that we learn for molar enthalpy of neutralisation. I was confused bc my tutor said that nwater=. nammonium salt produced which i was confused about because there is no water but then we learnt that neutralisation enthalpy calorimetry always has to have water in it and now im even more confusion
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 441
- Gender
- Undisclosed
- HSC
- 1998
OK, I understand your question.
It comes back to the different models of acids and bases.
The Arrhenius Model proposes that acids ionise to give H+ ions and bases ionise to give OH- ions. Therefore, under the Arrhenius Model, neutralisation will produce water molecules.
The Bronsted-Lowry Model proposes that acids are proton donors, and bases are proton receivers. Therefore, under the Bronsted-Lowry Model, there is no requirement to produce water molecules during neutralisation. All that has to happen is proton transfer.
It comes back to the different models of acids and bases.
The Arrhenius Model proposes that acids ionise to give H+ ions and bases ionise to give OH- ions. Therefore, under the Arrhenius Model, neutralisation will produce water molecules.
The Bronsted-Lowry Model proposes that acids are proton donors, and bases are proton receivers. Therefore, under the Bronsted-Lowry Model, there is no requirement to produce water molecules during neutralisation. All that has to happen is proton transfer.
synthesisFR
afterhscivemostlybeentrollingdonttakeitsrsly
would you say i should just treat ammonia as a special case: since basically all other acid base rxn produce water?OK, I understand your question.
It comes back to the different models of acids and bases.
The Arrhenius Model proposes that acids ionise to give H+ ions and bases ionise to give OH- ions. Therefore, under the Arrhenius Model, neutralisation will produce water molecules.
The Bronsted-Lowry Model proposes that acids are proton donors, and bases are proton receivers. Therefore, under the Bronsted-Lowry Model, there is no requirement to produce water molecules during neutralisation. All that has to happen is proton transfer.
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 441
- Gender
- Undisclosed
- HSC
- 1998
Ammonia is not the only case you will encounter in Chemistry of a Bronsted-Lowry base. Maybe it is time to revise the advantages of the Bronsted-Lowry Theory of Acids and Bases. You are probably going to get a question in the HSC to compare the advantages of Arrhenius and the Bronsted-Lowry theories.
synthesisFR
afterhscivemostlybeentrollingdonttakeitsrsly
this is still mod 6 right?Ammonia is not the only case you will encounter in Chemistry of a Bronsted-Lowry base. Maybe it is time to revise the advantages of the Bronsted-Lowry Theory of Acids and Bases. You are probably going to get a question in the HSC to compare the advantages of Arrhenius and the Bronsted-Lowry theories.
we haven't gotten upto this yet
Also thank u for all the help!
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 441
- Gender
- Undisclosed
- HSC
- 1998
Yes, this is one of the central big ideas of Mod.6this is still mod 6 right?
we haven't gotten up to this yet
Also thank u for all the help!


