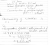The titration between potassium hydrogen phthalate and sodium hydroxide is a 1:1 stoichiometry. If the mean titration volume (titre) of sodium hydroxide to reach the endpoint is 26.2 mL against 25 mL of a 7.06 mM standard solution of potassium hydrogen phthalate, what is the concentration of sodium hydroxide (in mM)? Provide your answer to 3 significant figures.
Note: this is a hypothetical scenario and the concentrations may not match the previous question or the values used in your experiment.
I kept on doing this, and got the answer to be 7.404nM, but the quiz says its wrong, can someone provide me with the right answer and explanation? Thanks in advance
Note: this is a hypothetical scenario and the concentrations may not match the previous question or the values used in your experiment.
I kept on doing this, and got the answer to be 7.404nM, but the quiz says its wrong, can someone provide me with the right answer and explanation? Thanks in advance