-
Best of luck to the class of 2025 for their HSC exams. You got this! Let us know your thoughts on the HSC exams here
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
tuff ice table question (1 Viewer)
- Thread starter butan1ol
- Start date
kendricklamarlover101
Member
- Joined
- Oct 22, 2023
- Messages
- 79
- Gender
- Male
- HSC
- 2024
tf they doin to these reactions to make Keq 1000
Starting from the corrected question:
}{\Delta n(\text{B})})
...but this is only true when the volume of the system is constant, which is not the case here.
The simplest solution in such cases is to express the change row in chemical amounts (moles) so that stoichiometric ratios can be applied correctly.
In the following ICE table, the data from the question are shown in black; chemical amounts calculated from it are shown in bold; and, the change in [SO3] (shown in bold and coloured orange) has been calculated using the equation n = ne - ni.
n = ne - ni.
Now, determine the remaining changes (shown in bold in green) using stoichiometry:
The rest of the table can now be completed (shown in bold in purple).
These equilibrium concentrations can now be put into the expression for the equilibrium constant and the resulting equation solved:
^2\left(0.25C\right)} = \frac{0.25}{0.25C^2 \times C} = \frac{1}{C^3} \\ C^3 &= \frac{1}{1000} \\ C &= 0.1 \end{align*})
Thus, [SO3] = 0.1 mol L-1 in the initial mixture.
An ICE table can be constructed for this question by first setting the initial [SO3] to C mol L-1 (as this is the answer sought), and using (for each chemical substance) the equations:
- initial concentration (Ci) is related to its initial chemical amount (ni) by ni = CiVi, where Vi = 5.0 L.
- equilibrium concentration (Ce) is related to its equilibrium chemical amount (ne) by ne = CeVe, where Ve = 10.0 L.
...but this is only true when the volume of the system is constant, which is not the case here.
The simplest solution in such cases is to express the change row in chemical amounts (moles) so that stoichiometric ratios can be applied correctly.
In the following ICE table, the data from the question are shown in black; chemical amounts calculated from it are shown in bold; and, the change in [SO3] (shown in bold and coloured orange) has been calculated using the equation
| 2 SO2 (g) | + | O2 (g) | -----> <----- | 2 SO3 (g) |
Initial concentration, Ci / mol L-1 | 0.5 | | 0.25 | | C |
Initial chemical amount / mol ni = CiVi | 0.5 x 5.0 = 2.5 | | 0.25 x 5.0 = 1.25 | | C x 5.0 = 5C |
Change in chemical amount / mol | | | | | 2.5 - 5C |
Equilibrium chemical amount / mol ne = CeVe | | | | | 0.25 x 10.0 = 2.5 |
Equilibrium concentration, Ce / mol L-1 | | | | | 0.25 |
Now, determine the remaining changes (shown in bold in green) using stoichiometry:
2 SO2 (g) | + | O2 (g) | -----> <----- | 2 SO3 (g) | |
Ci / mol L-1 | 0.5 | | 0.25 | | C |
ni / mol | 2.5 | | 1.25 | | 5C |
| n(SO2) : n(SO3) = 2 : 2 = 1 = -1(2.5 - 5C) = 5C - 2.5 | | n(O2) : n(SO3) = 1 : 2 = 0.5 = -0.5(2.5 - 5C) = 2.5C - 1.25 | | 2.5 - 5C | |
ne / mol | | | | | 2.5 |
Ce / mol L-1 | | | | | 0.25 |
The rest of the table can now be completed (shown in bold in purple).
2 SO2 (g) | + | O2 (g) | -----> <----- | 2 SO3 (g) | |
Ci / mol L-1 | 0.5 | | 0.25 | | C |
ni / mol | 2.5 | | 1.25 | | 5C |
/ mol | n(SO2) : n(SO3) = 2 : 2 = 1 = -1(2.5 - 5C) = 5C - 2.5 | | n(O2) : n(SO3) = 1 : 2 = 0.5 = -0.5(2.5 - 5C) = 2.5C - 1.25 | | 2.5 - 5C |
ne = ni + / mol | 2.5 + (5C - 2.5) = 5C | | 1.25 + (2.5C - 1.25) = 2.5C | | 2.5 |
Ce = ne / 10.0 / mol L-1 | 0.5C | | 0.25C | | 0.25 |
These equilibrium concentrations can now be put into the expression for the equilibrium constant and the resulting equation solved:
Thus, [SO3] = 0.1 mol L-1 in the initial mixture.
Last edited:
Note: The initial mixture was not at equilibrium since
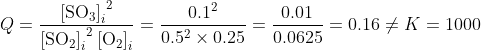
and, as Q < K, the system was moving right.
The doubling of the volume of the system is a disturbance that would cause a system at equilibrium to move to the left (on applying Le Chatelier's Principle and analysing as a decrease in pressure)... but this system was not at equilibrium when the volume change occurred.
In other words, we have an equilibrium system that is moving right when it experiences a Le Chatelier's Principle disturbance that would cause a shift left from the equilibrium position.
The cumulative effect of both factors on the equilibrium system cannot be determined qualitatively, for the following reasons:
We can take the impact of the volume change into account before any shift back to the equilibrium position occurs by calculating the reaction quotient as if the volume was changed before the initial mixture of gases was introduced. In other words, if the given initial concentrations are reduced by a factor of two (following the dilution formula, as the volume was doubled), then the reaction quotient initially would have been:
^2}{\left(\cfrac{0.250}{2}\right)^2\cfrac{0.250}{2}} = \frac{0.0500^2}{0.250^2\times 0.125} = 0.32 < K)
Hence, taking the effect of the volume change on the initial mixture into account increases the value of Q from 0.16 to 0.32, still far below K, and so the overall change is a shift to the right to return to equilibrium.
This is clear from the complete ICE table:
from which the reaction quotient could also be calculated by finding the concentrations using C = n / V:
}{V}\right]^2}{\left[\cfrac{n\left(\text{SO}_2\right)}{V}\right]^2\cfrac{n\left(\text{O}_2\right)}{V}} = \frac{\left(\cfrac{0.5}{10}\right)^2}{\left(\cfrac{2.5}{10}\right)^2\times\cfrac{1.25}{10}} = \frac{0.05^2}{0.25^2\times 0.125} = 0.32)
and, as Q < K, the system was moving right.
The doubling of the volume of the system is a disturbance that would cause a system at equilibrium to move to the left (on applying Le Chatelier's Principle and analysing as a decrease in pressure)... but this system was not at equilibrium when the volume change occurred.
In other words, we have an equilibrium system that is moving right when it experiences a Le Chatelier's Principle disturbance that would cause a shift left from the equilibrium position.
The cumulative effect of both factors on the equilibrium system cannot be determined qualitatively, for the following reasons:
- Suppose that the system is near to its equilibrium position when the volume change occurs:
- in this case, the volume change might lead to a new movement to the left, provided the effect of the volume change is sufficiently large
- but, if the effect of the volume change is small, the system may continue moving right.
- Now, suppose that the system started far from its equilibrium position (as is the case here, as Q = 0.16 and K= 1000):
- the cumulative result may still be a movement to the right unless the volume change has a very large effect.
- It is even possible for the two changes to nullify each other, in which case the system has reached equilibrium.
We can take the impact of the volume change into account before any shift back to the equilibrium position occurs by calculating the reaction quotient as if the volume was changed before the initial mixture of gases was introduced. In other words, if the given initial concentrations are reduced by a factor of two (following the dilution formula, as the volume was doubled), then the reaction quotient initially would have been:
Hence, taking the effect of the volume change on the initial mixture into account increases the value of Q from 0.16 to 0.32, still far below K, and so the overall change is a shift to the right to return to equilibrium.
This is clear from the complete ICE table:
2 SO2 (g) | + | O2 (g) | -----> <----- | 2 SO3 (g) | |
Ci / mol L-1 | 0.5 | | 0.25 | | C = 0.1 |
ni = 5Ci / mol | 2.5 | | 1.25 | | 5C = 0.5 |
1:1 5C - 2.5 = -2 | | 1 : 2 2.5C - 1.25 = -1 | | 2.5 - 5C = +2 | |
ne = ni + | 5C = 0.5 | | 2.5C = 0.25 | | 2.5 |
Ce = ne / 10 / mol L-1 | 0.05 | | 0.025 | | 0.25 |
from which the reaction quotient could also be calculated by finding the concentrations using C = n / V:
The numbers have clearly been selected to make the cubic equation one that is reasonable to solve. If you get a cubic equation that is not straight-forward in a chemistry question, you've either made a mistake or the question is not suitable for the HSC chemistry course.I got the answer, it's 0.1 mol L-1, but I used photomath to solve the cubic that arises. I guess my question is can you solve it without a cubic?
What cubic did you get? Can you see how your method (which found the correct answer) can be simplified to match the one that I have posted above?
Note, K = 1000 is not a particularly large equilibrium constant and this system is not "barely reversible"... looking at the ICE table above, you have a 10.0 L gas system with n(SO2) = 0.5 mol, n(O2) = 0.25 mol, and n(SO3) = 2.5 mol. This is a mixture in which the ratio of molecules of O2 : SO2 : SO3 is 1 : 2 : 10. Choosing a molecule from the system at random, the chance of it being an SO3 molecule is 10 / 13, or approximately 76.9%, so close to one quarter of the molecules in the mixture are one of the two reactants.i think it just means that the reaction is barely reversible it just occurs in one direction but theres like some small grains of the reactants left so they call it an equilibrium system
If the system were not an equilibrium but rather went to completion, then all of the limiting reagent would be consumed, producing 3.0 mol SO3 with a yield of 100%. Instead, had the system started with SO2 and O2 only, we have a yield of 83.3% and 16.7% of each reactant remains in the final equilibrium mixture.
You will encounter many systems where K is much larger than 103 or much smaller than 10-3. Though these will certainly see products or reactants favoured (respectively) in the composition of the equilibrium mixtures, these are not "barely reversible" systems - in fact, since the equilibrium state is dynamic, an expression like "barely reversible" is more applicable to a situation where the rate of the forward and reverse reactions is very close to zero, irrespective of the value of the equilibrium constant. In other words, it relates much more to the size of the activation energy barrier to the reactions than it does to the position of equilibrium.
FYI, the 2023 Independent Trial paper had a question on this point:
Consider the interconversion of diamond and graphite, two allotropes of the element carbon. At room temperature, the system can be represented by the equation:
It has been estimated that that it would take 3.0 x 1072 years for 1 g of diamond to change to 1 g of graphite.
This is an example of a spontaneous reaction that is (formally) an equilibrium system but where the activation energy barriers to both the forward and reverse reactions are so large that the system is effectively static.
I'm glad the explanation helped. I'll usually be less detailedshit bro you really went all out, thanks a lot for the explanation. in terms of the cubic that i got, the same as you, but i saw a x^3 term and immediately freaked out.
Don't freak out. It might seem odd, but the HSC chemistry course thinks you should be able to solve
but considers that it is not reasonable to ask you to use the quadratic formula! Anything with a power of 2 or higher you have to be able to solve by doing
or something similar.



