looking at the ksp on the data sheet can help with what's insoluble and what ppt'swhat is the probability that they'll ask us a long Mod 8 anion/cation testing question this year..... I do not think I will be good at gambling but I cannot absorb the solubility table for the life of me.
-
Finished your HSC? Check out our University Discussion, Non-School forums or help out next year's HSC students! -
YOU can help the next generation of students in the community! Share your trial papers and notes on our Notes & Resources page
Chemistry Predictions/Thoughts (2 Viewers)
- Thread starter twinkiecat
- Start date
pancake15
Member
- Joined
- Oct 26, 2024
- Messages
- 87
- Gender
- Female
- HSC
- 2024
syllabus mentions "nylon and polyesters" by name as an example in the same way they do for addition polymers, it specifies MODEL and compare structure, properties and uses of condensation polymers so bare minimum id say remember what types of monomers can be used + the linking functional groupDo we, I thought they usually give the condensation polymers and then tell you to show the monomer, but you have to remember the addition polymers for sure.
again, better safe than sorry though
randomuser246
Active Member
- Joined
- Oct 16, 2023
- Messages
- 131
- Gender
- Female
- HSC
- 2024
How do you answer questions about the effect of enthalpy and entropy on the reactions
person3024
Well-Known Member
- Joined
- May 11, 2023
- Messages
- 446
- Gender
- Female
- HSC
- 2024
legit i cant do thisHow do you answer questions about the effect of enthalpy and entropy on the reactions
pancake15
Member
- Joined
- Oct 26, 2024
- Messages
- 87
- Gender
- Female
- HSC
- 2024
Usually reference to the gibbs free formula resulting and the resulting gibbs free value (spontaneous/not) - which is basically same as the table (the one w entropy vs entropy and the boxes state spontaneity)How do you answer questions about the effect of enthalpy and entropy on the reactions
ie combustion of octane (GIVE EQUATION) - enthalpy of combustion is always negative, and since the number of gaseous molecules is larger in the products and the reactants (/no. of molecules of products overall) entropy is gained when combustion is undergone. Since enthalpy is negative and entropy is positive the reaction will be undergone spontaneously as gibbs free is negative, and then adjust accordingly depending on the reaction they give + enthalpy/entropy values (if they give both + the gibbs free value, basically it's the same ans but include the given values - ie BECAUSE enthalpy is (negative/positive value) and entropy is (negative/positive value), this yields the negative/positive value (whatever they provide) thus the rxn will be spontaneous/non spontaneous
if it's not clearly spontaneous/non spontaneous reference what temps it will be spontaneous at using the table, if they give gibbs free but its like ie small positive enthalpy and large positive entropy to give a negative gibbs free value, u can say rxn is entropy driven and vice versa if it doesnt adhere to the typical negative enthalpy positive entropy rule
Last edited:
pancake15
Member
- Joined
- Oct 26, 2024
- Messages
- 87
- Gender
- Female
- HSC
- 2024
legit i cant do this
notes from my summary attatched, idk if they'll be of any use but this is normally my approachHow do you answer questions about the effect of enthalpy and entropy on the reactions
Attachments
-
3.2 MB Views: 88
-
1.8 MB Views: 65
pancake15
Member
- Joined
- Oct 26, 2024
- Messages
- 87
- Gender
- Female
- HSC
- 2024
idk prolly given 2021 was the hardest paper, but the 2019 additional questions has some pretty hard oneswhat paper had the hardest multis, 2021 ?
person3024
Well-Known Member
- Joined
- May 11, 2023
- Messages
- 446
- Gender
- Female
- HSC
- 2024
lifesavernotes from my summary attatched, idk if they'll be of any use but this is normally my approach
pancake15
Member
- Joined
- Oct 26, 2024
- Messages
- 87
- Gender
- Female
- HSC
- 2024
compd
New Member
- Joined
- Nov 2, 2024
- Messages
- 10
- Gender
- Undisclosed
- HSC
- N/A
2020 was the hardest paper, not 2021idk prolly given 2021 was the hardest paper, but the 2019 additional questions has some pretty hard ones
femboys4life
Active Member
- Joined
- Aug 18, 2024
- Messages
- 181
- Gender
- Female
- HSC
- 2024
is skeletal formula accepted
Yes, there are many sample responses in HSC papers where they use this formis skeletal formula accepted
shoulfer
Well-Known Member
- Joined
- Feb 7, 2023
- Messages
- 342
- Gender
- Male
- HSC
- 2024
yep but I would only use it within a response not as your final answer, for your final answer as a molecule id say to stick to structuralis skeletal formula accepted
ManifestationIsKey
Active Member
- Joined
- Oct 25, 2024
- Messages
- 153
- Gender
- Male
- HSC
- 2024
Yes it is accepted. It is still a universal way of representing the structure, but as @shoulfer said, it would be preferred to stick to structural, but I doubt they would penalise for having a skeletal structureis skeletal formula accepted
tiredcloud
New Member
- Joined
- Oct 30, 2024
- Messages
- 14
- Gender
- Female
- HSC
- 2024
Where can I find the 2019 NESA practice questions, they don't seem to be on their website?
Here it is : https://educationstandards.nsw.edu....al-sample-hsc-questions.pdf?MOD=AJPERES&CVID=Where can I find the 2019 NESA practice questions, they don't seem to be on their website?
pineapple546
New Member
- Joined
- Oct 20, 2024
- Messages
- 1
- Gender
- Female
- HSC
- 2024
willy_g0706
Member
- Joined
- Aug 27, 2023
- Messages
- 28
- Gender
- Male
- HSC
- 2024
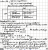
can do the calculation but H+ in water = 10-7. that little of an acid will not change the pHView attachment 45638
help!! how do u solve this??