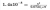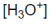I don’t rlly get this question. What does percent solution by mass mean? And how does the density relate to the working out?
Novocaine, C13H21O2N2CI, is the salt of the base procaine and HCI. The ionisation constant is 7x10^-6. Is a solution of novocaine acidic or basic? Furthermore, what are the concentrations of H3O+ OH- and pH of a solution of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL
Novocaine, C13H21O2N2CI, is the salt of the base procaine and HCI. The ionisation constant is 7x10^-6. Is a solution of novocaine acidic or basic? Furthermore, what are the concentrations of H3O+ OH- and pH of a solution of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL