-
YOU can help the next generation of students in the community! Share your trial papers and notes on our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Module 8 - Spectra Help!!! (1 Viewer)
- Thread starter Nash__
- Start date
IR spectra: shows key functional groups. Strong sharp absorbance at 2959, 2934, 2862 cm^-1 (C-H stretch). Strong sharp absorbance at 1718 cm^-1 (C=O). This indicates the molecule is a ketone or aldehyde.
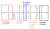
1H NMR: shows the number of hydrogen environments and their chemical environments. There are 5 (maybe 6 --> see the 1.3 ppm below) unique hydrogen environments in this molecule:
- 2.4 ppm (2H and triplet - 2H neighbours)
- 2.15 ppm (3H and singlet - 0H neighbours)
- 1.6 ppm (2H and quintet - 4H neighbours)
- 1.3 ppm (4H and multiplet. It is probably 2 signals overlapping this is why it looks choppy and not symmetrical on each side like the other signals ---> so that gives you 2 x CH2 which accounts for the 4H)
- 0.9 ppm (3H and triplet - 2H neighbours)
The 3H singlet means there is a CH3 connected to the C=O, connecting it like that will section that CH3 off from the rest of the molecule and therefore that explains the 0 neighbours.
The structure is most likely (heptan-2-one) (just as a comment this kind of question wouldn't show up in an exam, because they will always give you clean spectrums where the signals are all visible. This is just a problem of sometimes spectrums in real life aren't as clean or nice as they are laid out in a textbook and recognising this is something that just takes experience)
1H NMR: shows the number of hydrogen environments and their chemical environments. There are 5 (maybe 6 --> see the 1.3 ppm below) unique hydrogen environments in this molecule:
- 2.4 ppm (2H and triplet - 2H neighbours)
- 2.15 ppm (3H and singlet - 0H neighbours)
- 1.6 ppm (2H and quintet - 4H neighbours)
- 1.3 ppm (4H and multiplet. It is probably 2 signals overlapping this is why it looks choppy and not symmetrical on each side like the other signals ---> so that gives you 2 x CH2 which accounts for the 4H)
- 0.9 ppm (3H and triplet - 2H neighbours)
The 3H singlet means there is a CH3 connected to the C=O, connecting it like that will section that CH3 off from the rest of the molecule and therefore that explains the 0 neighbours.
The structure is most likely (heptan-2-one) (just as a comment this kind of question wouldn't show up in an exam, because they will always give you clean spectrums where the signals are all visible. This is just a problem of sometimes spectrums in real life aren't as clean or nice as they are laid out in a textbook and recognising this is something that just takes experience)

Wow. Thank you so much!! Yeah I guess that multiplet was tripping me up as I was trying to construct something with 5 hydrogen environments and simultaneously trying to meet the condition for a group of 4 hydrogens (i was going into cyclic compounds, it was messy!)
Just confirming, would you say that there are still 5 hydrogen environments however there are 2 which have almost the same chemical shift hence they merge?
Just confirming, would you say that there are still 5 hydrogen environments however there are 2 which have almost the same chemical shift hence they merge?
It would be 6 hydrogen environments, although they are overlapping and it appears like there are 5. If you had a higher magnetic field NMR instrument it would be able to split those two overlapping signalsWow. Thank you so much!! Yeah I guess that multiplet was tripping me up as I was trying to construct something with 5 hydrogen environments and simultaneously trying to meet the condition for a group of 4 hydrogens (i was going into cyclic compounds, it was messy!)
Just confirming, would you say that there are still 5 hydrogen environments however there are 2 which have almost the same chemical shift hence they merge?
Oops sorry, I meant to say 6 hydrogen environments. Thanks manIt would be 6 hydrogen environments, although they are overlapping and it appears like there are 5. If you had a higher magnetic field NMR instrument it would be able to split those two overlapping signals

