Menomaths
Exaı̸̸̸̸̸̸̸̸lted Member
- Joined
- Jul 9, 2013
- Messages
- 2,373
- Gender
- Male
- HSC
- 2013
2011 HSC question from Quanta to Quarks:
23592U+10n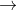 14056Ba+9236Kr+410n
14056Ba+9236Kr+410n
Explain why energy is released in this reaction.
The answer says:
Energy is released because the mass of the products of this reaction is less than the mass
of the reactants.
This ‘missing’ mass is converted to energy through the equation E = mc
When I add up the mass of U and the neutron (I'm assuming I'm not supposed to add the mass of the neutron?) it equals the mass of RHS(including the 4 neutrons)
23592U+10n
Explain why energy is released in this reaction.
The answer says:
Energy is released because the mass of the products of this reaction is less than the mass
of the reactants.
This ‘missing’ mass is converted to energy through the equation E = mc
When I add up the mass of U and the neutron (I'm assuming I'm not supposed to add the mass of the neutron?) it equals the mass of RHS(including the 4 neutrons)

