do u just google the qs and find the paper? bit confusedHello, this probably won't be the best attempt to help but I hope it helps
The answer to question 10 is 680 mm Hg, i.e. (C).
For question 3:
50 mL. Initially 200 mL, finally 150 mL (100 mL CO2 + 50 mL leftover O2).
I sadly have no idea about question 5
Questions 4 and 6 are from the Sydney Boys High School 2019 year 11 Chemistry half yearly paper. If you can by any chance access the answers of that paper, they might help you with those questions.
This seems like a combination of questions from different sources. For example, question 3 is from a chemistry olympiad and questions 4 and 6 are from the SBHS paper I mentioned above.
-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
pllssss helllpppp yr 11 q mod 2 urgennttttt!!! the later photo is of higher priority. (1 Viewer)
- Thread starter yashbb
- Start date
My knowledge of Chemistry is sadly limited beyond this point, but I'll try my best to provide working out for this question as soon as I can!could you tel me how you got the answer for q10?
EDIT: looks like it’s been already provided. I will still include working out regardless:

I hope this helps!
It depends, sometimes (particularly with subjects I've taken) I might be able to recognise the paper(s) in which the question appears. When that's not the case, I may search for the questions online and determine their source.do u just google the qs and find the paper? bit confused
Last edited:
u remember y11 qs wtfMy knowledge of Chemistry is sadly limited beyond this point, but I'll try my best to provide working out for this question as soon as I can!
It depends, sometimes (particularly with subjects I've taken) I might be able to recognise the paper(s) in which the question appears. When that's not the case, I may search for the questions online and determine their source.
For Q 10, we are told that the pressure of the gas in the sealed tube is 615 mmHg. This refers to the pressure when the mercury levels in the two arms are equal. Since both the sealed section of gas and the atmosphere each exert a force on the mercury, the levels adjust until these forces are equal. We can see that the outside pressure from the atmosphere is greater than 615 mmHg as the atmospheric pressure is forcing the the mercury down the tube and up the sealed arm, which will continue to rise until the sealed gas is sufficiently compressed to have a pressure to equal to the atmosphere and thus for the forces on the mercury in each arm to be the same. At that position shown in the diagram, the height difference is 65 mm, indicating a pressure difference of 65 mmHg from the known pressure.
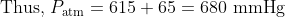
Eagle Mum
Well-Known Member
- Joined
- Nov 9, 2020
- Messages
- 603
- Gender
- Female
- HSC
- N/A
Q5 is C
Multiply the volume (0.300 L) by the molar concentration (0.25 mol/L) for sodium sulphate and add the product of the volume (0.200L), molar concentration (0.08 mol/L) and number of sulfate ions released per aluminium sulphate molecule (3), to obtain 0.123 moles of sulphate ions in 0.5 L which is 0.246 mol/L. However, since the molar concentration of aluminium is given only to one s.f. (no trailing zeros after the ‘8’), the correct answer should also only be to one s.f. (ie. 0.2 which is option C).
0.3 x 0.25 + 0.2 x 0.08 x 3 = 0.123 moles
0.3 + 0.2 = 0.5 litres
molar concentration = 0.123 / 0.5 = 0.246 mol/L (0.2 mol/L to 1 s.f.)
Multiply the volume (0.300 L) by the molar concentration (0.25 mol/L) for sodium sulphate and add the product of the volume (0.200L), molar concentration (0.08 mol/L) and number of sulfate ions released per aluminium sulphate molecule (3), to obtain 0.123 moles of sulphate ions in 0.5 L which is 0.246 mol/L. However, since the molar concentration of aluminium is given only to one s.f. (no trailing zeros after the ‘8’), the correct answer should also only be to one s.f. (ie. 0.2 which is option C).
0.3 x 0.25 + 0.2 x 0.08 x 3 = 0.123 moles
0.3 + 0.2 = 0.5 litres
molar concentration = 0.123 / 0.5 = 0.246 mol/L (0.2 mol/L to 1 s.f.)
