To continue with the line of incomplete answers,
A porous divider is similar to a salt bridge in that it plays the same function in a cell; to allow for the balancing of the charge distribution in the cell. It allows ions in the solution to pass between the half cells, allowing each half cell to maintain a neutral charge.
For the other one:
Dependent Variable: Colour of solution/Change in colour of solution
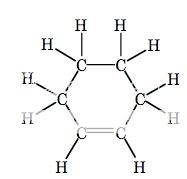
I will copy and paste the structural formula from my notes:
Cyclohexene
One factor which justifies the use of this alkene is the fact that it is a liquid at room temperature, making it much easier to contain, and work with in a fume cupboard. Also, cyclohexene is a clear liquid, which allows the colour change to be easily observed, adding to the reliability of the experiment.
Question:
How can biomass be used as an alternative source as opposed to fossil fuels for the petrochemical industry. (In our half yearly, this was an assess question work 8 marks)